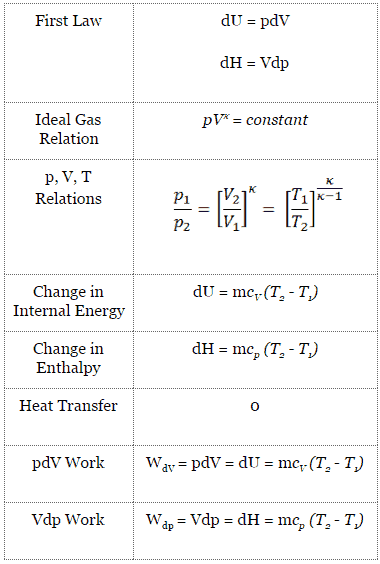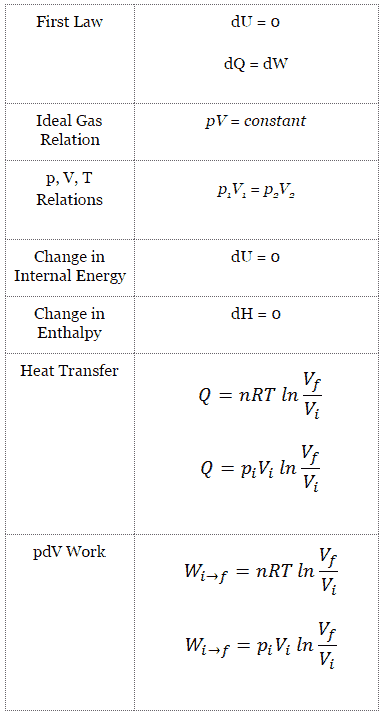Thermodynamic Processes
 A thermodynamic process is defined as a change from one equilibrium macrostate to another macrostate. The initial and final states are the defining elements of the process. During such a process, a system starts from an initial state i, described by a pressure pi, a volume Vi and a temperature Ti , passes through various quasistatic states to a final state f, described by a pressure pf, a volume Vf , and a temperature Tf. In this process energy may be transferred form or into the system and also work can be done by or on the system. One example of a thermodynamic process is increasing the pressure of a gas while maintaining a constant temperature. In following section, there are examples of thermodynamic processes that are of highest importance in engineering of heat engines.
A thermodynamic process is defined as a change from one equilibrium macrostate to another macrostate. The initial and final states are the defining elements of the process. During such a process, a system starts from an initial state i, described by a pressure pi, a volume Vi and a temperature Ti , passes through various quasistatic states to a final state f, described by a pressure pf, a volume Vf , and a temperature Tf. In this process energy may be transferred form or into the system and also work can be done by or on the system. One example of a thermodynamic process is increasing the pressure of a gas while maintaining a constant temperature. In following section, there are examples of thermodynamic processes that are of highest importance in engineering of heat engines.
Types of Thermodynamic Processes
Reversible Process
In thermodynamics, a reversible process is defined as a process that can be reversed by inducing infinitesimal changes to some property of the system, and in so doing leaves no change in either the system or surroundings. During reversible process the entropy of the system does not increase and the system is in thermodynamic equilibrium with its surroundings.
Irreversible Process
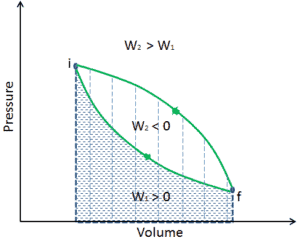
In thermodynamics, an irreversible process is defined as a process that cannot be reversed, process, that cannot return both the system and the surroundings to their original conditions.
During irreversible process the entropy of the system increases.
Cyclic Process
A process that eventually returns a system to its initial state is called a cyclic process. At the conclusion of a cycle, all the properties have the same value they had at the beginning. For such a process, the final state is the same as the initial state, and so the total internal energy change must be zero.
It must be noted, according to the second law of thermodynamics, not all heat provided to a cycle can be transformed into an equal amount of work, some heat rejection must take place. The thermal efficiency, ηth, of any heat engine as the ratio of the work it does, W, to the heat input at the high temperature, QH. ηth = W/QH.
See also: Reversible Process
See also: Irreversible Process
See also: Cyclic Process
Isentropic Process
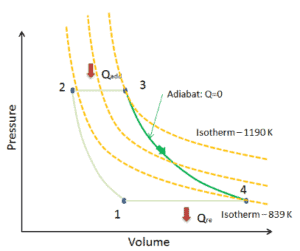
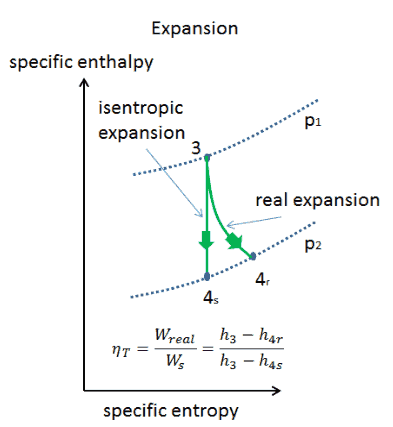
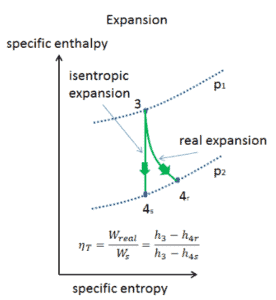
An isentropic process is a thermodynamic process, in which the entropy of the fluid or gas remains constant. It means the isentropic process is a special case of an adiabatic process in which there is no transfer of heat or matter. It is a reversible adiabatic process. An isentropic process can also be called a constant entropy process. In engineering such an idealized process is very useful for comparison with real processes.
See also: Isentropic Process
Adiabatic Process
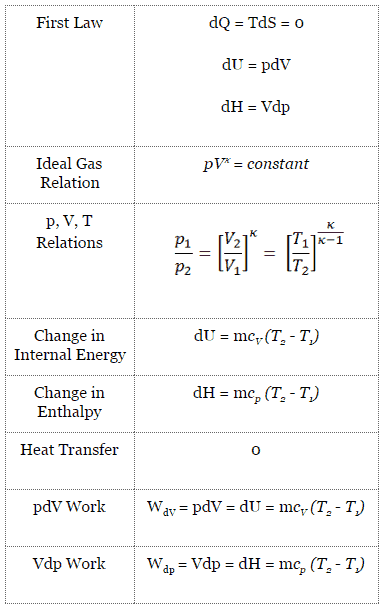
An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated. In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes. In these rapid processes, there is not enough time for the transfer of energy as heat to take place to or from the system.
In real devices (such as turbines, pumps, and compressors) heat losses and losses in the combustion process occur, but these losses are usually low in comparison to overall energy flow and we can approximate some thermodynamic processes by the adiabatic process.
See also: Adiabatic Process
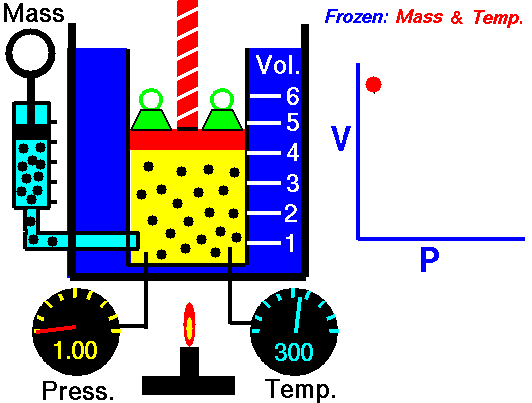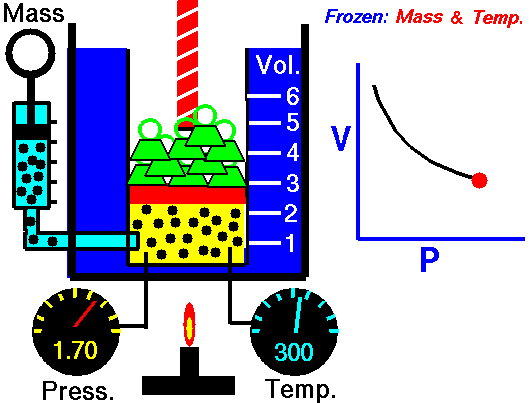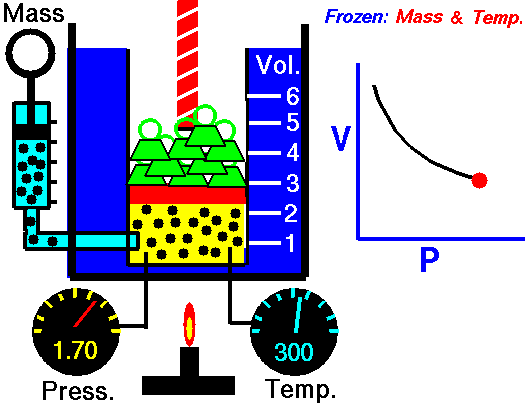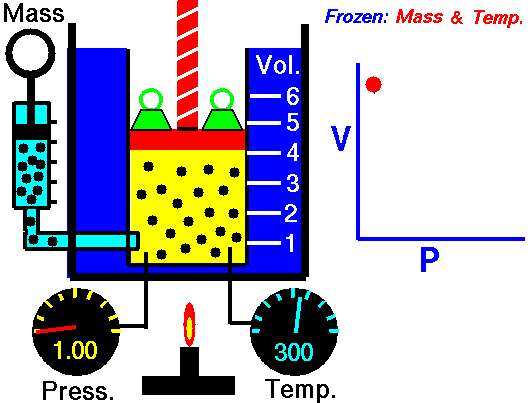
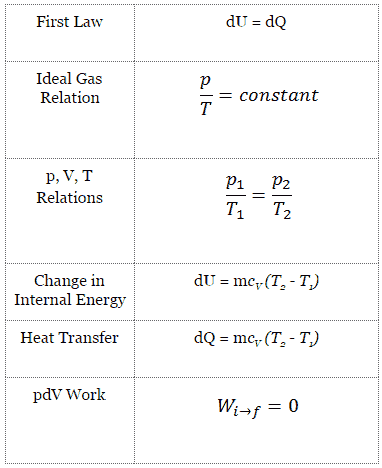
Isothermal Process
An isothermal process is a thermodynamic process, in which the temperature of the system remains constant (T = const). The heat transfer into or out of the system typically must happen at such a slow rate in order to continually adjust to the temperature of the reservoir through heat exchange. In each of these states the thermal equilibrium is maintained.
For an ideal gas and a polytropic process, the case n = 1 corresponds to an isothermal (constant-temperature) process. In contrast to adiabatic process , in which n = κ and a system exchanges no heat with its surroundings (Q = 0; ∆T≠0), in an isothermal process there is no change in the internal energy (due to ∆T=0) and therefore ΔU = 0 (for ideal gases) and Q ≠ 0. An adiabatic process is not necessarily an isothermal process, nor is an isothermal process necessarily adiabatic.
See also: Isothermal Process
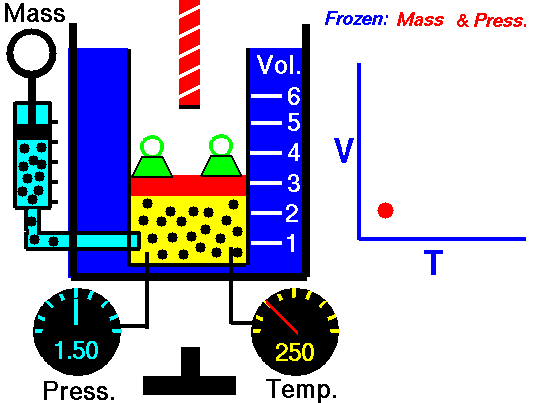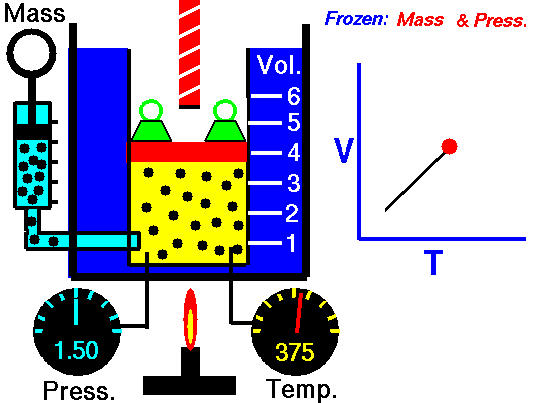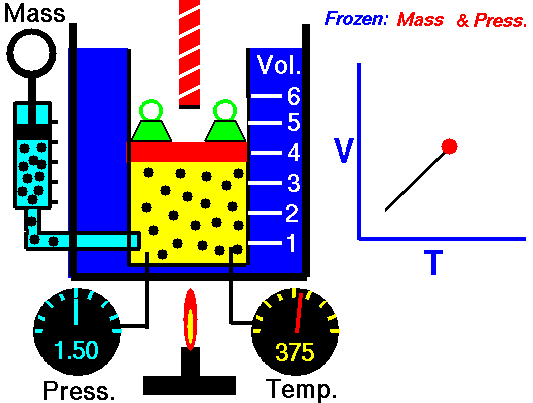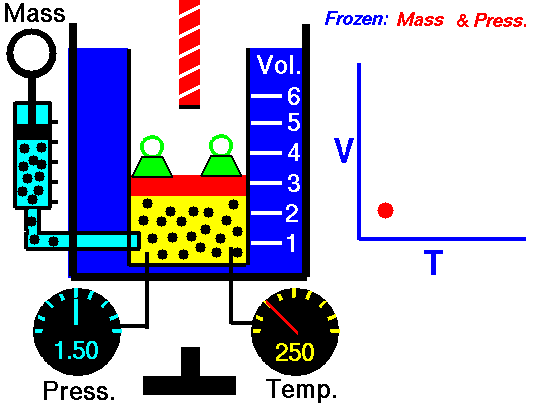
Isobaric Process
An isobaric process is a thermodynamic process, in which the pressure of the system remains constant (p = const). The heat transfer into or out of the system does work, but also changes the internal energy of the system.
Since there are changes in internal energy (dU) and changes in system volume (∆V), engineers often use the enthalpy of the system, which is defined as:
H = U + pV
In many thermodynamic analyses it is convenient to use the enthalpy instead of the internal energy. Especially in case of the first law of thermodynamics.
In engineering, both very important thermodynamic cycles (Brayton and Rankine cycle) are based on two isobaric processes, therefore the study of this process is crucial for power plants.
See also: Isobaric Process
Isochoric Process
An isochoric process is a thermodynamic process, in which the volume of the closed system remains constant (V = const). It describes the behavior of gas inside the container, that cannot be deformed. Since the volume remains constant, the heat transfer into or out of the system does not the p∆V work, but only changes the internal energy (the temperature) of the system.
In engineering of internal combustion engines, isochoric processes are very important for their thermodynamic cycles (Otto and Diesel cycle), therefore the study of this process is crucial for automotive engineering.
See also: Isochoric Process
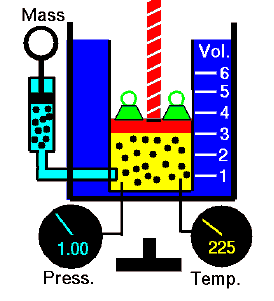
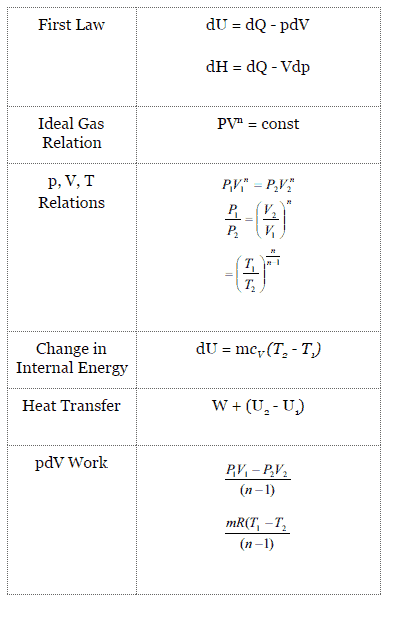
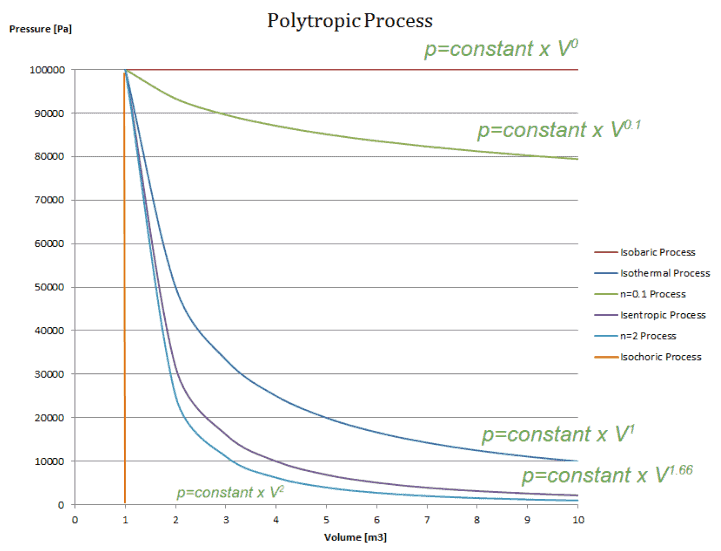
Polytropic Process
A polytropic process is any thermodynamic process that can be expressed by the following equation:
pVn = constant
The polytropic process can describe gas expansion and compression which include heat transfer. The exponent n is known as the polytropic index and it may take on any value from 0 to ∞, depending on the particular process.
See also: Polytropic Process
Throttling Process – Isenthalpic Process
A throttling process is a thermodynamic process, in which the enthalpy of the gas or medium remains constant (h = const). In fact, the throttling process is one of isenthalpic processes. During the throttling process no work is done by or on the system (dW = 0), and usually there is no heat tranfer (adiabatic) from or into the system (dQ = 0). On the other the throttling process cannot be isentropic, it is a fundamentally irreversible process. Characteristics of throttling process:
- No Work Transfer
- No Heat Transfer
- Irreversible Process
- Isenthalpic Process
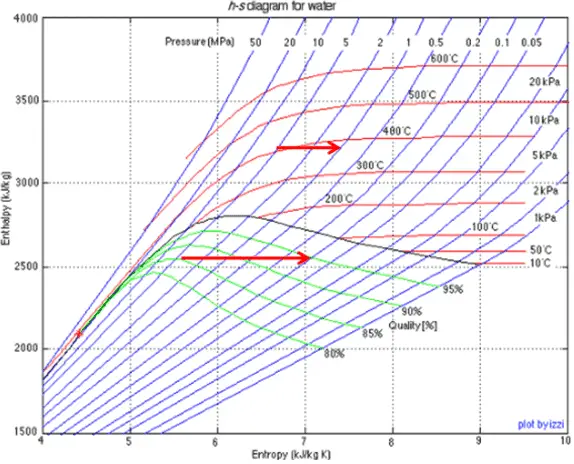
Throttling of the wet steam is also associated with conservation of enthalpy. But in this case a reduction in pressure causes an increase in vapor quality.
See also: Throttling Process
We hope, this article, Thermodynamic Process, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.