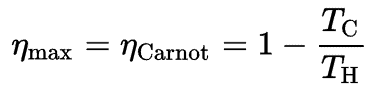Various Statements of the Law
The second law of thermodynamics may be expressed in many specific ways. Each statement expresses the same law. Listed below are three that are often encountered.
Before these statements, we have to remind the work of a French engineer and physicist, Nicolas Léonard Sadi Carnot advanced the study of the second law by forming a principle (also called Carnot’s rule) that specifies limits on the maximum efficiency any heat engine can obtain.
Kelvin-Planck Statement of the Second Law
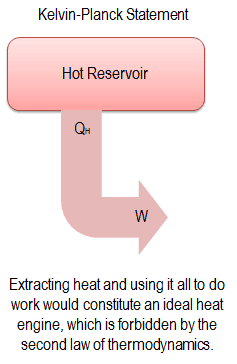 “It is impossible to construct a device which operates on a cycle and produces no other effect than the production of work and the transfer of heat from a single body”.
“It is impossible to construct a device which operates on a cycle and produces no other effect than the production of work and the transfer of heat from a single body”.
This statement operates with the term “thermal reservoir” or “single reservoir”. A reservoir is a large object, in which the temperature remains constant while energy is extracted. Such a system can be approximated in a number of ways—by the earth’s atmosphere, large bodies of water like lakes, oceans and so on.
The Kelvin–Planck statement does not exclude the existence of a system, that develops a net amount of work from a heat transfer extracted from a thermal reservoir. According to this statement, a system undergoing a cycle cannot develop a positive net amount of work from a heat transfer extracted from a thermal reservoir.
We hope, this article, Kelvin-Planck Statement of the Second Law, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.